Casting Light on Deposition: The Opposite of Sublimation
Confused about the opposite of sublimation? You’re not alone! Deposition is when a gas turns directly into a solid, and it’s not the same as sublimation. We’ll make it clear so that you can fully comprehend this phase transition and its functioning.
Both processes involve phase transitions, but while sublimation requires heat to move from solid to gas, deposition releases heat as the gas condenses into a solid.
Understanding Sublimation and Deposition
Sublimation and deposition might sound like complex terms, but they’re actually quite simple. Sublimation is when a solid turns directly into a gas without becoming a liquid first. Deposition is the opposite: a gas turns directly into a solid.
Let’s break it down:
- Sublimation: It happens when a solid gains enough energy to skip the liquid stage and go straight to a gas. A common example is dry ice. At -78.5°C, dry ice (solid carbon dioxide) turns directly into carbon dioxide gas. You might have seen this at parties or in school experiments.

- Deposition: It occurs when a gas loses enough energy to become a solid without passing through the liquid phase. Frost forming on a cold surface is a good example. Water vapor in the air turns directly into ice crystals on surfaces like windows or grass.
Even though these processes may seem unusual, they are happening around us all the time. Recognizing and explaining these changes in your daily life can be aided by understanding their workings.
Now that we clearly understand sublimation let’s explore its antithesis.
Scientific Explanation of Phase Changes
Now that we’ve seen sublimation and deposition in action, let’s take a closer look at the science behind these fascinating phase changes. Understanding the underlying principles can help us appreciate why these processes occur and how they differ from other phase changes like melting or evaporation.
Energy and Temperature Changes
- Sublimation: For sublimation to happen, a solid needs to gain enough energy to overcome the forces holding its molecules in a fixed position. This usually occurs at specific temperatures and pressures. For instance, dry ice sublimes at -78.5°C because, at this temperature, the molecules have enough energy to break free from their solid structure and become a gas.
- Deposition: Deposition requires gas to lose enough energy so that its molecules can settle into a fixed position, forming a solid. This typically happens when the gas comes into contact with a very cold surface. The energy loss allows the gas molecules to slow down and bond together, forming a solid. Frost on a window is a good example, where water vapor in the air deposits directly as ice crystals on the cold glass.
Comparison with Other Phase Changes
- Melting and Freezing: These are more familiar phase changes where a solid turns into a liquid (melting) and a liquid turns into a solid (freezing). Unlike sublimation and deposition, these processes involve an intermediate liquid phase.
- Evaporation and Condensation: Evaporation is when a liquid turns into a gas, and condensation is when a gas turns into a liquid. These processes also differ from sublimation and deposition by involving a liquid state.
Why These Phase Changes Occur
- Molecular Movement: The key to understanding these phase changes lies in the movement and energy of molecules. In a solid, molecules are closely packed together and vibrate in place. When they gain enough energy, they can break free and move apart, turning into a gas (sublimation). Conversely, gas molecules lose energy and slow down, bonding together to form a solid (deposition).
- Pressure and Temperature Conditions: Sublimation and deposition usually occur under specific conditions of pressure and temperature. For example, water can sublimate at temperatures below freezing when the air is dry enough to allow water molecules to escape from the ice without becoming liquid first.
Understanding Phase Changes
|
Phase Change |
Energy Change |
Temperature and Pressure Conditions |
Example |
|---|---|---|---|
|
Sublimation |
Solid gains energy |
Occurs at specific low temperatures and low pressures |
Dry ice (solid CO₂) sublimes at -78.5°C |
|
Deposition |
Gas loses energy |
Occurs when gas comes into contact with a cold surface |
Frost forming on a window |
|
Melting |
Solid gains energy |
Occurs at the melting point of the substance |
Ice melting into water |
|
Freezing |
Liquid loses energy |
Occurs at the freezing point of the substance |
Water freezing into ice |
|
Evaporation |
Liquid gains energy |
Occurs at the boiling point or below if energy is sufficient |
Water evaporating into steam |
|
Condensation |
Gas loses energy |
Occurs when gas is cooled below its condensation point |
Steam condensing into water |
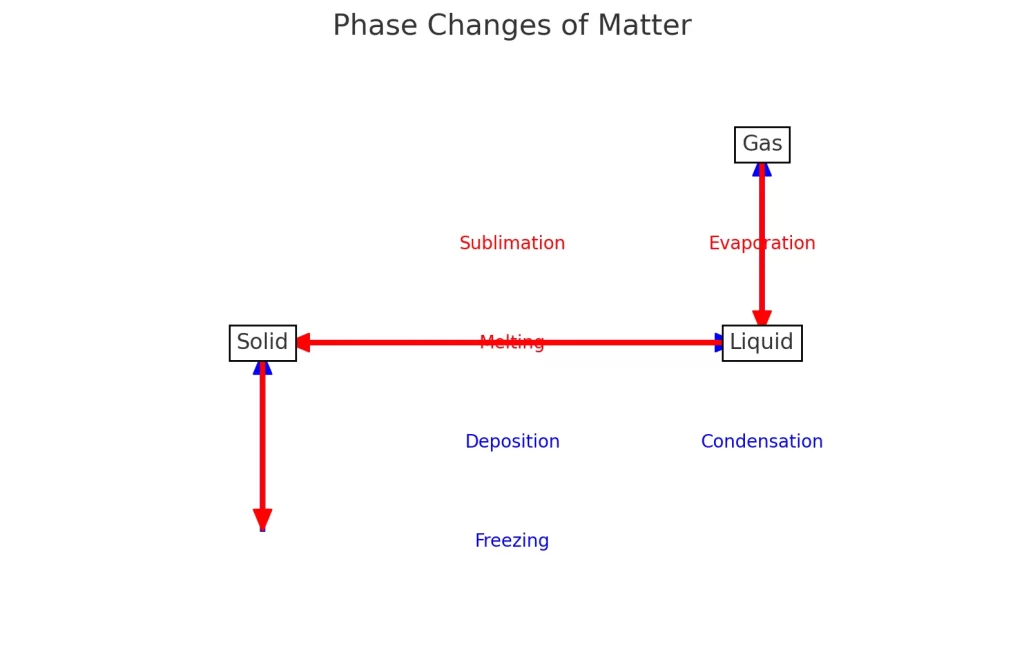
Chart showing the phase changes of matter. It illustrates how substances transition between solid, liquid, and gas states through processes like sublimation, deposition, melting, freezing, evaporation, and condensation.
- Sublimation: Solid to Gas
- Deposition: Gas to Solid
- Melting: Solid to Liquid
- Freezing: Liquid to Solid
- Evaporation: Liquid to Gas
- Condensation: Gas to Liquid
The Enigmatic World of Deposition
In material science, deposition is the process that starkly contrasts to sublimation. Deposition, often called “opposite sublimation,” transforms a substance from a gas directly into a solid without passing through the liquid state. This intriguing process occurs when gaseous particles lose energy and transition into a solid state, typically on a surface or substrate.
Applications of Deposition
Deposition has found a multitude of applications across various industries. Let’s explore the critical areas where this process plays a pivotal role:
1. Thin Film Technology
In thin film technology, deposition processes create light layers of materials on various substrates. This technology is crucial in manufacturing semiconductors, optical coatings, and photovoltaic cells. By depositing precise layers of materials, engineers can control the properties of these thin films, influencing their conductivity, reflectivity, and more.
2. Protective Coatings
Deposition is also widely employed in the creation of protective coatings. These coatings are essential in industries such as aerospace, where they shield critical components from harsh environmental conditions. Deposition techniques ensure these coatings adhere firmly to the surface, providing durability and longevity.
3. Pharmaceuticals
In the pharmaceutical industry, deposition plays a vital role in drug formulation. It enables the creation of controlled-release drug delivery systems, where active pharmaceutical ingredients are deposited onto carriers. This allows for the gradual release of medication within the body, ensuring optimal therapeutic effects.
The Role of Deposition in Nanotechnology
Nanotechnology, a field at the cutting edge of scientific exploration, heavily relies on deposition processes. Researchers use these techniques to fabricate nanoscale structures and devices with remarkable precision. From nanowires to quantum dots, deposition opens the door to many possibilities in the nanoworld.
Beyond the Basics: A Universe of Phase Changes
Sublimation and deposition are just two pieces of a much larger puzzle – the world of phase changes! Let’s explore some additional concepts to broaden our understanding:
- A Phase Change Family: Sublimation and deposition are not alone. Melting (solid to liquid) and vaporization (liquid to gas) are typical phase changes. Understanding these transformations helps us predict and explain various phenomena in different fields.
- Not Quite Opposites: It’s important to note that sublimation and deposition aren’t perfect opposites. While they involve opposite state changes, they occur under distinct pressure and temperature conditions.
- For instance, dry ice sublimates at room temperature because it has a shallow sublimation point. Conversely, deposition typically requires colder temperatures or lower pressure for gas particles to condense directly into a solid.
Thinking Outside the Lab:
Understanding phase changes goes beyond textbooks. It has practical applications in various fields:
- Chemistry: Phase changes are crucial in distillation (purifying liquids) and crystallization (forming pure solids from solutions).
- Meteorology: Understanding how water vapor condenses and sublimates helps explain cloud formation and precipitation (rain, snow).
- Food Science: As mentioned earlier, freeze-drying utilizes sublimation to preserve food.
Sublimation and Fusion
Understanding both sublimation and fusion gives a clearer picture of how different materials respond to changes in temperature and pressure. By recognizing the conditions and applications for each, you can better appreciate their roles in science and industry.
Sublimation
- Definition: Sublimation is the process where a solid changes directly into a gas without passing through the liquid phase. This occurs under specific conditions of temperature and pressure.
- Examples: Dry ice sublimating into carbon dioxide gas, and the gradual disappearance of snow even when the temperature remains below freezing.
- Applications: Sublimation is used in freeze-drying food, preserving biological samples, and in sublimation printing for high-quality, durable prints on various materials.

Fusion (Melting)
- Definition: Fusion, or melting, is the process where a solid turns into a liquid when heat is applied, reaching its melting point.
- Examples: Ice melting into water at 0°C, and metals like gold melting when heated to high temperatures.
- Applications: Melting is essential in cooking, metalworking, and manufacturing processes where materials need to be liquefied and reshaped.
Comparison of Sublimation and Fusion
- Phase Change Path: Sublimation skips the liquid phase, going directly from solid to gas, while fusion involves a transition from solid to liquid.
- Energy Requirements: Both processes require energy input, but the amount and type of energy differ. Sublimation requires sufficient energy to overcome the forces holding the solid together and transition directly to gas, while fusion requires energy to break the solid structure and form a liquid.
- Pressure and Temperature Conditions: Sublimation typically occurs under low pressure and at temperatures below the substance’s triple point. Fusion occurs at a specific temperature (melting point) for a given pressure.
- Practical Uses: Both processes have practical uses, but they are applied in different contexts. Sublimation is valuable in preservation and printing, while fusion is crucial in various manufacturing and everyday processes.
Sublimation vs. Fusion: Key Differences
| Aspect | Sublimation | Fusion |
|---|---|---|
| Phase Change Path | Solid → Gas (skips liquid) | Solid → Liquid |
| Energy Requirements | High energy to break solid bonds directly | Moderate energy to melt solid |
| Conditions | Low Pressure, below triple point | At melting point for given pressure |
| Practical Uses | Preservation, Printing | Manufacturing, Cooking |
Ready to Bring Science to Life?
At Subli Genius Print, we master the art and science of sublimation, helping you harness these processes in practical, innovative ways.
As we examine the duality of sublimation and deposition, we gain profound insights into the fascinating processes that shape materials science.
Share Your Experiences
Have you noticed frost forming on windows or experienced freezer burn? What intrigued you most about these phenomena? We’d love to hear your stories!




