Sublimation in Nature: How It Shapes Our World
Sublimation, a term often associated with the realm of science, is a fascinating natural process that we encounter more often than we realize.
In its simplest definition, sublimation is the transition of a substance directly from the solid to the gas phase, without passing through the intermediate liquid phase. This might sound complex, but it’s a process that’s happening all around us, and even within us.
| Example | Environment / Type | Process Observed | Why It Matters |
|---|
| Snow on Himalayas | Nature / Mountains | Solid → Gas | Supplies atmospheric moisture, influences local precipitation, and sustains ecosystems |
| Ice cubes in freezer | Everyday Life / Home | Solid → Gas | Demonstrates sublimation indoors, affecting food storage and household experiments |
| Mothballs | Household / Storage | Solid → Gas (odor release) | Protects textiles and clothing from insects using sublimation of chemicals |
| Frost on leaves | Nature / Outdoors | Gas → Solid (deposition) | Shapes microclimates, affects plant surfaces, and contributes to frost formation |
| Dry ice in spa | Wellness / Recreational | Solid → Gas (fog effect) | Creates calming visual effects and enhances relaxation experiences |
| Polar ice caps | Nature / Glaciers | Solid → Gas | Reduces ice mass, contributes to atmospheric moisture, and impacts climate patterns |
| Glaciers | Nature / High-altitude | Solid → Gas | Influences glacier mass loss and downstream water supply |
| Aromatherapy diffusers | Wellness / Tech | Solid → Gas | Releases essential oils into the air for therapeutic and sensory benefits |
- Direct phase change: Sublimation is when a solid turns straight into gas without becoming liquid.
- Where it happens: Most common in cold, low-pressure environments like mountains, glaciers, and polar regions.
- Everyday examples: Ice cubes in freezers, mothballs, and dry ice all sublimate in daily life.
- Environmental impact: Influences the water cycle, glacier mass, frost formation, and local ecosystems.
- Wellness & tech uses: Dry ice in spas, aromatherapy diffusers, and visual fog effects all rely on sublimation.
How to Observe Sublimation in Nature and Everyday Life
Sublimation isn’t just a concept, it’s something you can actually see for yourself! Here’s a simple step-by-step guide:
What You’ll Need:
- Ice cubes or a freezer
- Mothballs (optional)
- Dry ice (for supervised or controlled use)
- A cold outdoor environment (for frost observation)
- Optional: Thermometer, clear container or tray
Steps:
- Observe Sublimation in Ice Cubes
Place ice cubes on an open tray in your freezer. Over time, you’ll notice they slowly shrink without melting into water. That’s sublimation—the ice turning directly into gas. - Observe Sublimation in Mothballs
Put a few mothballs in a small container. As they slowly disappear, they release a gas that protects clothing from insects. Sublimation in action! - Observe Sublimation Using Dry Ice
In a safe, controlled environment, place dry ice in a container. Watch as the fog forms while it turns directly from solid to gas. It’s a dramatic example of sublimation. - Observe Frost Formation
On a cold, clear night, check leaves or surfaces outside. Water vapor from the air can deposit directly as frost (gas → solid), showing sublimation in reverse.
Why It Matters:
These simple observations help you understand how sublimation affects the natural world, from glaciers and snow in the mountains to everyday phenomena like frost or ice shrinking in your freezer. Plus, it’s a fun way to connect science with the world around you.
Sublimation in the Water Cycle
The water cycle, a continuous movement of water on, above, and below the surface of the Earth, is a process we learn about in our early school years. But did you know that sublimation plays a crucial role in this cycle?
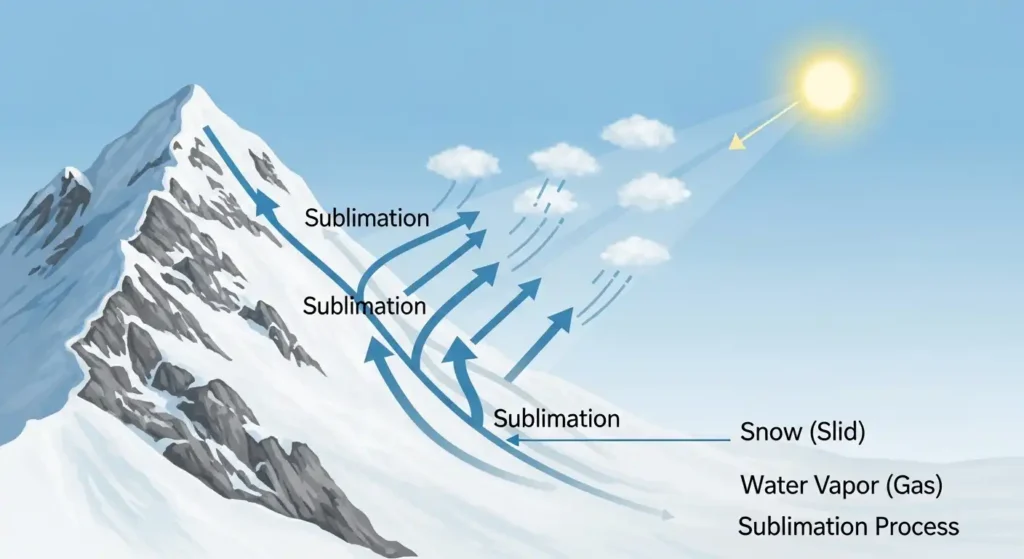
Sublimation in the water cycle primarily occurs in frigid regions where snow and ice are common. Instead of melting into water and then evaporating, the solid water, or ice, sublimates directly into water vapor in the air. This is especially common in the frosty peaks of mountains where the air pressure is low, and the temperatures are below freezing.
A real-life example of this is the dry, cold winds that sweep across the snow-capped peaks of the Himalayas. The snow doesn’t melt and flow down the slopes. Instead, it vanishes into thin air, literally. This is sublimation in action. Check out Sublimation and the Water Cycle | U.S. Geological Survey (usgs.gov)

How Does Sublimation Contribute to Natural Phenomena?
- Snow and Ice Disappearance: In cold regions, sublimation causes snow and ice to disappear without melting. This happens because the sun’s energy is strong enough to turn the solid ice or snow directly into water vapor.
- Frost Formation: On cold, clear nights, water vapor in the air can change directly into frost on surfaces, bypassing the liquid stage.
- Drying Out: In arid environments, sublimation helps dry out materials like rocks and soil, influencing the landscape and ecosystem.
What Role Does Sublimation Play in the Water Cycle?
Sublimation is an important part of the water cycle, especially in polar regions and high-altitude areas:
- Polar Ice Caps: Sublimation helps reduce the size of polar ice caps. Even in freezing temperatures, ice can turn into water vapor and enter the atmosphere.
- Glaciers: Similar to polar ice, glaciers lose mass through sublimation. This process contributes to the movement and melting of glaciers.
- Atmospheric Moisture: Sublimation adds water vapor directly to the atmosphere, which can eventually lead to precipitation elsewhere.
Sublimation in Everyday Life
While sublimation might seem like a process that’s restricted to the scientific world or the great outdoors, it’s a phenomenon that’s present in our everyday lives.
One of the most common examples of sublimation we encounter is in our freezers. Have you ever noticed how ice cubes shrink when left in the freezer for a long time? That’s sublimation! The ice cubes are slowly turning from solid to gas over time, even in the cold environment of your freezer.
Another example is the use of mothballs. These small balls of chemical pesticide and deodorant sublimate over time, transitioning from a solid state to a gas, releasing a smell that keeps moths and other insects at bay.
Sublimation’s Role in Nature
What is sublimation, and how does it occur in nature?
Sublimation is the process where a solid changes directly into a gas without passing through the liquid stage. This occurs in nature under specific conditions, such as low pressure and cold temperatures. For example, snow or ice turns directly into water vapor in a high-altitude environment.
Why is sublimation important in natural processes?
Sublimation plays a crucial role in various natural processes. For instance, it contributes to the loss of ice and snow in polar and mountainous regions, affecting local ecosystems and water supplies. Additionally, it impacts the Earth’s climate by influencing the atmospheric moisture content.

Sublimation in the Water Cycle
How does sublimation fit into the water cycle?
Sublimation is a part of the water cycle where ice and snow transform directly into water vapor. This typically happens in cold, dry environments like high-altitude mountains and polar regions. Sublimation adds moisture to the atmosphere, which eventually returns to the surface as precipitation.
How does snow turn into vapor without melting?
Snow turns into vapor without melting through the process of sublimation. This occurs when the temperature and pressure conditions favor the transition of a solid directly to a gas. In nature, this can be observed in arid, cold climates where the air is dry, and the sun’s energy provides enough heat for sublimation to occur.
Real-world examples of Sublimation
What happens to snow on mountains in winter?
In winter, snow on mountains can undergo sublimation due to the dry air and strong sunlight at high altitudes. As a result, the snow slowly turns into water vapor, contributing to the overall moisture in the atmosphere. This process is more pronounced on sunny days and south-facing slopes.
How does frost form on leaves through sublimation?
Frost forms on leaves when water vapor in the air sublimates directly onto cold surfaces. This occurs typically during clear nights when temperatures drop significantly, causing the water vapor to deposit as ice crystals on leaves and other surfaces. This is an example of deposition, the reverse of sublimation.
Are there other natural examples of sublimation?
Yes, sublimation also occurs in deserts where solid ice can turn directly into vapor without melting due to the dry air and intense heat. Additionally, in the polar regions, the ice caps and glaciers experience sublimation, especially during the summer months when temperatures rise.
Sublimation in the Wilderness
Sublimation doesn’t just occur in our homes or the water cycle; it’s also a significant process in the wilderness, particularly in cold, high-altitude environments.
For outdoor enthusiasts, understanding sublimation can enhance your appreciation of nature’s wonders. For instance, when hiking or camping in mountainous regions, you might notice that the snow or ice around you seems to disappear, even though the temperature is below freezing. This is sublimation at work!
Sublimation also has a profound impact on the environment and wildlife. It contributes to the formation and movement of glaciers, which are vital water sources for many species. Moreover, sublimation can affect weather patterns, creating unique microclimates in high-altitude habitats.
The Beauty of Sublimation
Sublimation, while a scientific process, also contributes to the breathtaking beauty of our natural world. This section will take you on a visual journey through some of the most stunning phenomena caused by sublimation.
One such phenomenon is the formation of snowflakes. Each snowflake begins as a tiny ice crystal, and as it falls to the ground, it experiences sublimation and re-sublimation, growing into a unique, intricate design. This process is responsible for the diverse and beautiful shapes of snowflakes that we all admire.
Another example is the creation of frostflowers in polar sea ice. These delicate ice structures form when the air is much colder than the underlying sea ice, causing the moisture to sublimate and then freeze into exquisite, flower-like formations
Sublimation and Wellness
Sublimation also finds its place in the realm of wellness and relaxation. The process contributes to creating soothing and rejuvenating experiences that many of us enjoy.
One such example is the use of dry ice in spa treatments. Dry ice, which is solid carbon dioxide, sublimates at room temperature. When used in a controlled manner, the fog produced by dry ice can create a calming and mystical atmosphere, enhancing the relaxation experience.
Another instance is the use of aromatherapy diffusers. Many of these devices use the process of sublimation to disperse the essential oil particles into the air, filling the room with a pleasant and therapeutic aroma.
Final Thoughts:
We hope this exploration of sublimation in nature has sparked your curiosity and deepened your appreciation for the intricate processes that shape our world. At Subli Genius Print, we believe in the power of knowledge to enhance our experiences, whether it’s understanding the science behind our natural world or improving our wellness practices.
As we continue to explore the intersection of nature, wellness, and outdoor living, we invite you to join us on this journey. Check out our other blog posts for more insights and inspiration.
Before you go, we have a question for you: How has this newfound understanding of sublimation changed your perspective on the natural world? Share your thoughts in the comments below. We’d love to hear from you!






One Comment