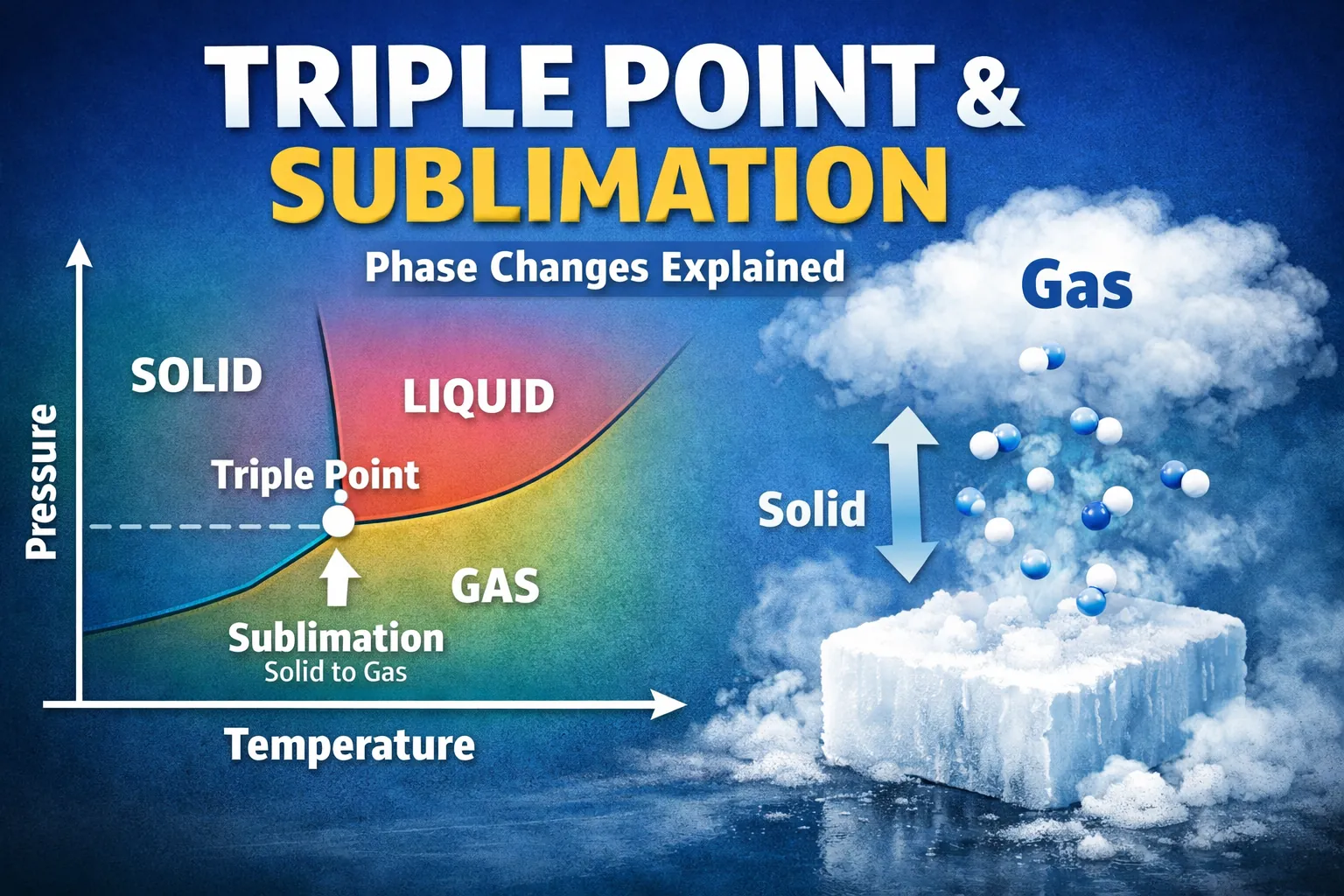
Triple Point and Sublimation: Why Pressure Determines Phase Change
Why does dry ice turn directly into gas without melting? The answer lies in a precise thermodynamic condition known as the triple point. This unique combination of temperature and pressure determines whether a substance can exist as a solid, liquid, or gas and whether sublimation is even possible.
Understanding the relationship between the triple point and sublimation reveals how phase transitions truly work.
By examining pressure boundaries and phase diagrams, we can clearly see why some substances bypass the liquid state entirely under certain conditions.
Introduction to Phase Transitions
Matter exists in three primary states: solid, liquid, and gas. A phase transition occurs when a substance changes from one state to another due to changes in temperature or pressure. Common phase transitions include melting (solid to liquid), freezing (liquid to solid), evaporation (liquid to gas), and condensation (gas to liquid).
These transitions occur because temperature affects molecular energy, while pressure influences how closely molecules are forced together. When either variable changes sufficiently, the balance between phases shifts, causing matter to transform.
Among all phase transitions, one condition stands out for its uniqueness: the triple point. This specific combination of temperature and pressure allows all three phases of a substance to exist in equilibrium simultaneously. Understanding this condition is essential for explaining how sublimation occurs.
What Is the Triple Point?
The triple point is the exact temperature and pressure at which a substance’s solid, liquid, and gas phases coexist in thermodynamic equilibrium.
At this point:
- The rates of melting and freezing are equal
- The rates of evaporation and condensation are equal
- The rates of sublimation and deposition are equal
Because these processes balance perfectly, the substance can exist in all three states at the same time without any net change.
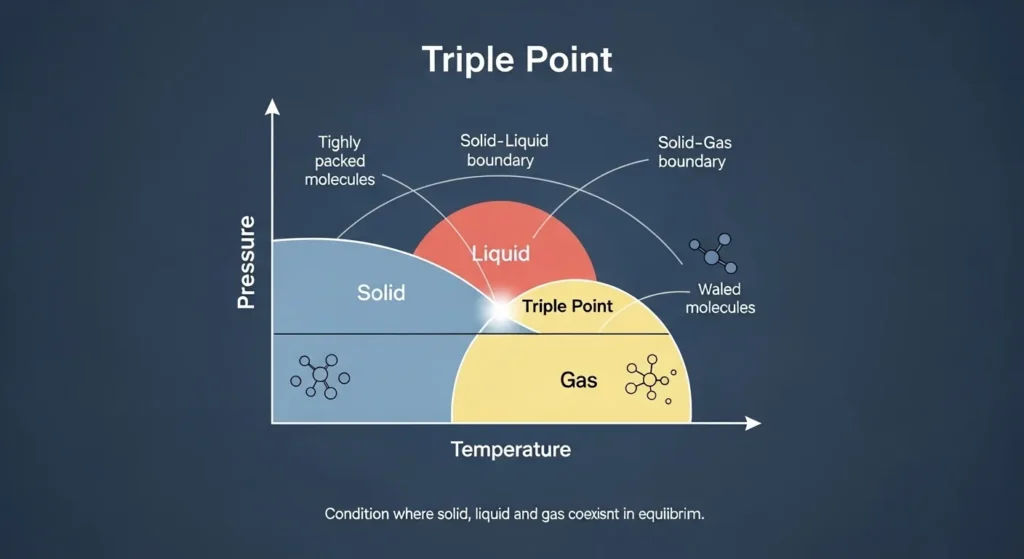
Each substance has a unique triple point determined by its molecular structure and intermolecular forces. For example, the triple point of water occurs at 0.01°C and 611.657 pascals. At this precise condition, ice, liquid water, and water vapor can coexist in equilibrium.
The triple point serves as a fundamental reference in thermodynamics because it defines the boundary conditions between phase transitions.
What Is Sublimation?
Sublimation is the direct transition of a substance from the solid phase to the gas phase without passing through the liquid phase.
This process occurs when molecules in a solid gain enough energy to escape directly into the vapor phase. Unlike melting, sublimation does not require the substance to become liquid first.
For sublimation to occur:
- The pressure must be below the substance’s triple-point pressure
- The temperature must be high enough to provide sufficient molecular energy
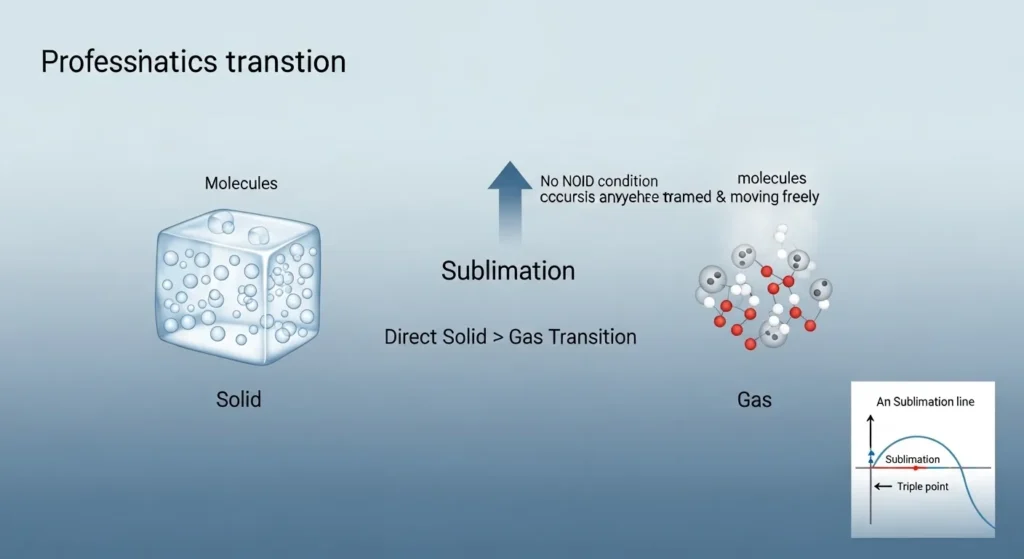
If the pressure is above the triple-point pressure, the substance must melt before it can become a gas. Therefore, sublimation is only possible under specific thermodynamic conditions.
Sublimation is a direct result of how temperature and pressure interact on a substance’s phase diagram.
If you want a deeper scientific breakdown of how this works, you can explore our full guide on the sublimation process,
How the Triple Point Determines When Sublimation Occurs
The triple point establishes the minimum pressure at which a liquid phase can exist.
Below the triple-point pressure, the liquid state is no longer stable. Under these conditions, a substance cannot melt into a liquid. Instead, it transitions directly between solid and gas.
This is why sublimation occurs only when pressure is lower than the triple-point pressure.
If pressure increases above the triple point:
- The solid will melt before vaporizing
- Sublimation will no longer be the dominant phase transition
If pressure decreases below the triple point:
- The liquid phase disappears from the phase diagram
- Sublimation becomes the only pathway between solid and gas
In this way, the triple point acts as a boundary marker that determines whether sublimation is thermodynamically possible.
Phase Diagram Explanation (Simple Overview)
A phase diagram is a graphical representation of the states of matter under different temperature and pressure conditions.
The diagram contains three main regions:
- Solid region
- Liquid region
- Gas region
These regions are separated by phase boundary lines:
- Solid–liquid boundary (melting/freezing line)
- Liquid–gas boundary (evaporation/condensation line)
- Solid–gas boundary (sublimation/deposition line)
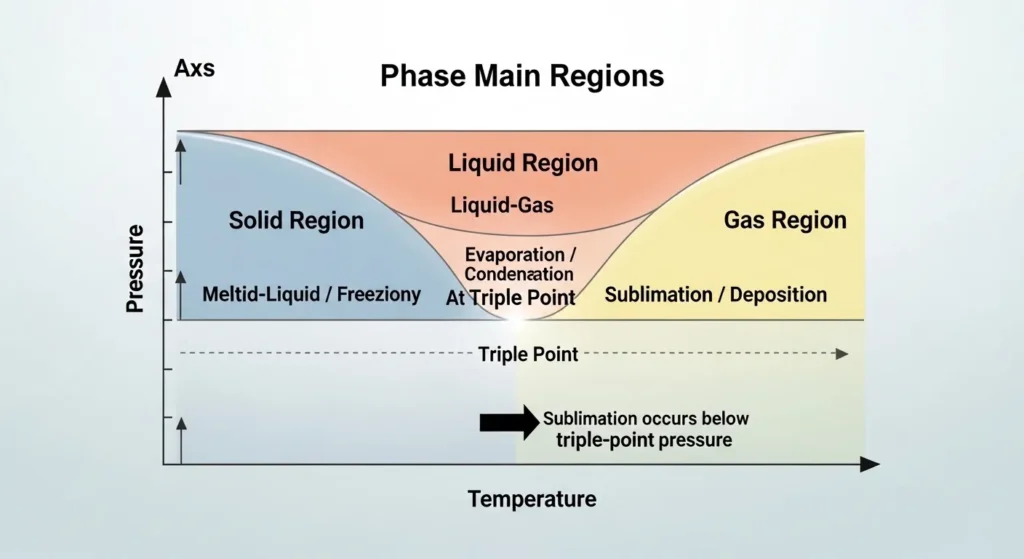
The triple point is the single point where all three boundary lines intersect.
Below the triple-point pressure, the liquid region disappears. This means that increasing temperature causes a solid to move directly into the gas region which is sublimation.
Thus, the phase diagram visually demonstrates how the triple point controls the conditions required for sublimation.
Real Scientific Examples (Water, CO₂, Iodine)
Water (H₂O)
The triple point of water occurs at 0.01°C and 611.657 pascals. Below this pressure, water cannot exist as a liquid. Ice exposed to sufficiently low pressure can sublimate directly into water vapor.
This principle is important in atmospheric science and high-altitude environments.
This principle is important in atmospheric science, freeze-drying technology, and high-altitude environments. You can also explore more real-world cases in our article on sublimation in nature, where we explain how these transitions happen outside the lab.
Carbon Dioxide (CO₂)
Carbon dioxide has a triple point at −56.6°C and 5.11 atmospheres.
At standard atmospheric pressure (1 atmosphere), carbon dioxide cannot exist as a liquid. Because this pressure is below its triple-point pressure, solid carbon dioxide (dry ice) sublimates directly into gas at −78.5°C.
This makes carbon dioxide one of the most commonly observed examples of sublimation.
Iodine (I₂)
Iodine sublimates at relatively low temperatures, producing a characteristic violet vapor.
Its triple point occurs at a pressure higher than typical atmospheric conditions. As a result, iodine can transition directly from solid to gas when gently heated under normal pressure.
This behavior is frequently demonstrated in laboratory experiments to illustrate sublimation clearly.
FAQ:
Why does sublimation only occur below the triple-point pressure?
Below the triple-point pressure, the liquid phase cannot exist. When temperature increases under these conditions, a solid transitions directly into gas because melting is no longer thermodynamically possible.
Is the triple point the same for every substance?
No. Each substance has a unique triple point determined by its molecular structure and intermolecular forces. The exact temperature and pressure vary from one material to another.
Does sublimation happen at normal atmospheric pressure?
It depends on the substance. If atmospheric pressure is below the substance’s triple-point pressure, sublimation can occur. For example, carbon dioxide sublimates at standard atmospheric pressure because its triple-point pressure is higher than 1 atmosphere.